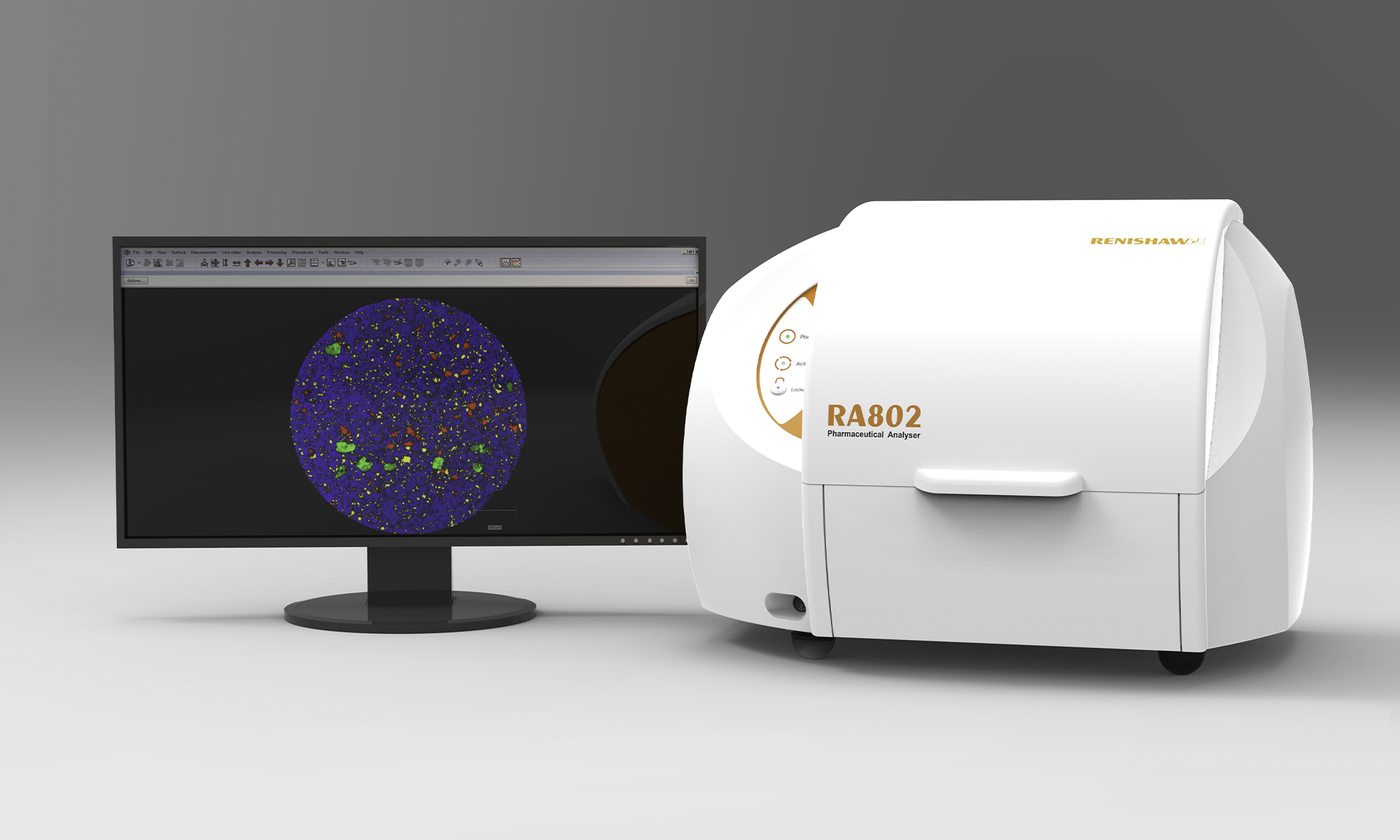
RA802 Pharmaceutical analyser
A rapid Raman imaging system designed to help scientists tackle formulation challenges and expedite drug development.
The RA802 Pharmaceutical analyser is a dedicated high-speed Raman system designed for pharmaceutical analysis. It can generate comprehensive chemical images of sample surfaces in as little as 5 minutes using Renishaw's unique LiveTrack™ and StreamLine™ technologies.
It can be used to better understand pharmaceutical formulations to speed up drug development or perform root cause analysis.
Features
The RA802 Pharmaceutical analyser enables you to generate chemical images up to 150 times faster than conventional methods, whilst maintaining focus – ensuring pharmaceutical tablet images are of high quality.
Chemical components can be highlighted by colour on Raman images and characterised using particle statistics. This gives users a tool to understand how each component is distributed throughout the sample and rationalise real-world performance between different batches and formulations.
For more detailed information, download the RA802 product note.
Focus and resolution
LiveTrack focus tracking technology automatically maintains sample focus, ensuring you acquire the highest quality chemical images across the whole sample. Spatial resolution can be as high as 1 µm per pixel – allowing scientists to understand the local inhomogeneity of their sample.
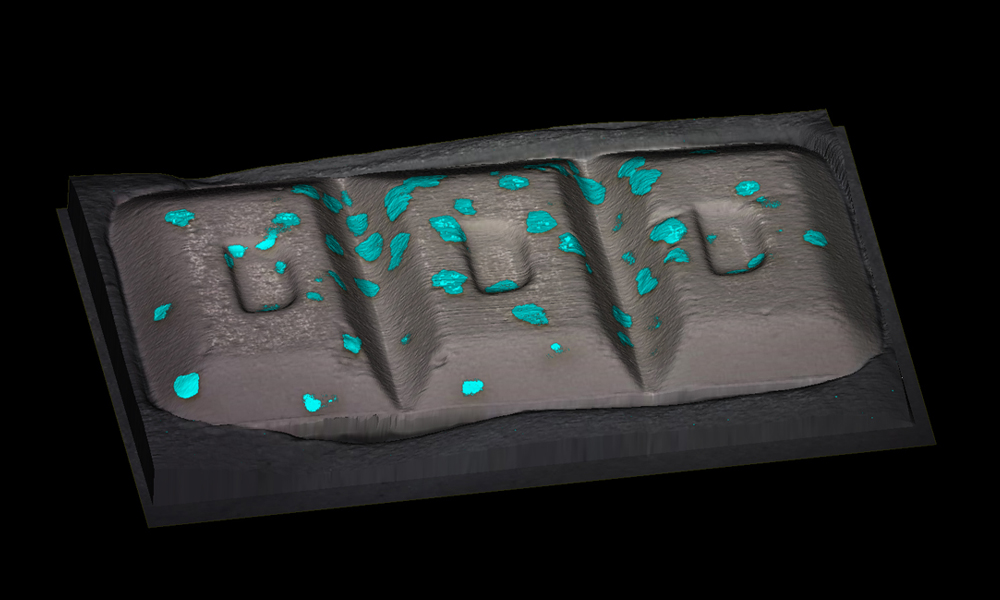
Speed of analysis
StreamLine rapid imaging technology increases the imaging speed to 1,500 spectra per second which makes analysis faster than ever and prevents sample damage. A whole tablet can be mapped in as little as 5 minutes at low-resolution, or just 2 hours at the highest resolution.

Batch measurement
Batch measurement automation enables the user to save time by programming multiple samples to run sequentially. Renishaw can provide bespoke tablet holders specific to the users' samples. These hold the samples securely in place whilst the system analyses each sample in turn.
Applications
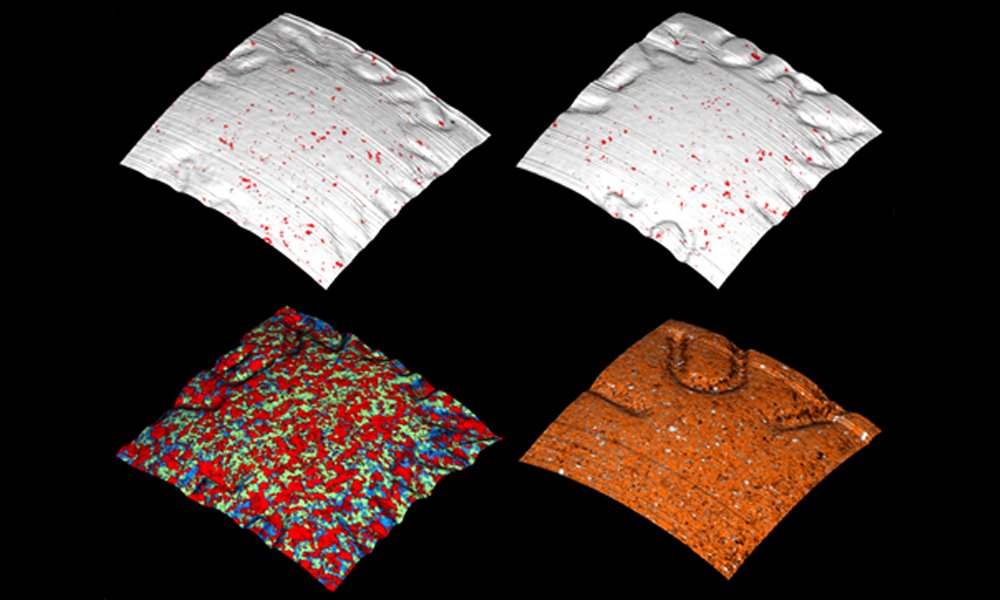
Identifying counterfeit medicines
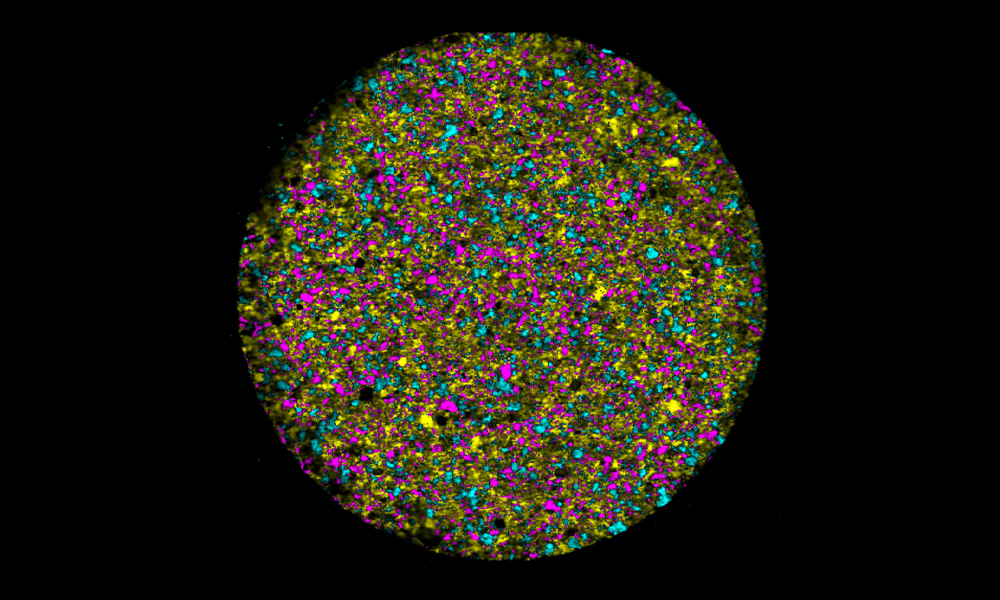
Raman mapping can generate high-resolution (1 µm2 per pixel) chemical images of complex blends of materials, such as tablets. These images enable you to study differences not only in chemical components but also the formulation's microstructure – an important way of identifying counterfeits.

Deformulating drugs
In this white paper we look at reverse engineering existing drug products using Raman imaging. We collected thousands of Raman spectra across the surface of a sample to determine the microstructure of a formulation. Each pixel contains all the chemical information for that sample point.
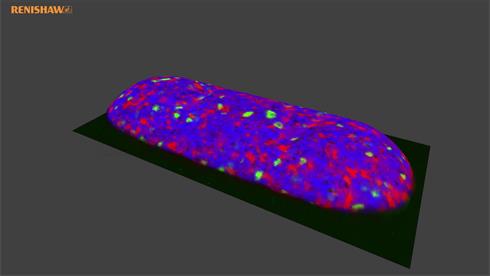
Maintain focus in real
time with LiveTrack
RA802 analyser uses LiveTrack™ automated focus tracking technology to acquire, in real-time, accurate and repeatable spectra and topography from samples with extensive variations in height. Create stunning 3D images of uneven, curved or rough surfaces without the need for pre-scanning. Watch the video to see an example.
Want to find out more?
Your local representative will be happy to help with your enquiry.
You can contact them by completing a form or sending an email.
Get our latest updates
Stay up-to-date with our latest news, webinars, application notes and product launches delivered directly to your inbox.
Downloads: RA802 Pharmaceutical analyser
-
 Brochure: RA802 Pharmaceutical analyser
Brochure: RA802 Pharmaceutical analyser
Designed exclusively for the pharmaceutical industry, the RA802 rapidly obtains detailed information on the distribution of chemical species.
-
 Application note: New methods for determining content uniformity of formulations
Application note: New methods for determining content uniformity of formulations
Using the RA802 to monitor the uniformity of a drug product’s contents to ensure that the correct dose is delivered every time.
-
 Application note: Rapid polymorph identification with the RA802 Pharmaceutical Analyser
Application note: Rapid polymorph identification with the RA802 Pharmaceutical Analyser
Characterising the polymorphism of active pharmaceutical ingredients when developing a commercially viable product.
-
 Application note: Using Raman spectroscopy to tackle polymorphism, an industry problem
Application note: Using Raman spectroscopy to tackle polymorphism, an industry problem
This note looks at incidences of polymorphism in drug development including advantages and challenges.
-
 White paper: Finding fakes – using Raman imaging to identify counterfeit medicines
White paper: Finding fakes – using Raman imaging to identify counterfeit medicines
Raman spectroscopy is highly specific and well-suited to identifying counterfeits which often use similar components to the reference product.
-
 Technical note: LiveTrack focus-tracking technology
Technical note: LiveTrack focus-tracking technology
LiveTrack focus-tracking technology maintains optimum focus for both white light and Raman imaging to give stunning 3D images.
Specifications
Parameter | Value |
| Laser wavelength | 785 nm Laser power: >150 mW at sample. Innovative StreamLine technology enables higher laser power use without sample damage |
| Spectral range | 100 cm-1 to 3250 cm-1 |
| Spectral dispersion | 2 cm-1 pixel-1 |
| Minimum image pixel size | 1 µm |
| Field of view | Macro ~21 mm × 16 mm (with zoom options) Micro ~ 330 µm × 250 µm (with zoom options) |
| Focusing | Macro – Manual or pre-defined Micro – Automatic (LiveTrack) or manual |
| Data collection speed | Over 1500 spectra/s |
| Maximum sample size | ~ (110 mm × 90 mm × 25 mm) – fits 96 well plate |
| Power, voltage | 100 – 240 VAC ± 10%, 50/60Hz, 100 W maximum |
| Dimensions | 720 mm (W) × 502 mm (H) × 535 mm (D) |
| Mass (not including computer) | 54 kg |
| Laser class | Class 1 laser product complies with IEC60825-1. CE marked |
| Software | Renishaw WiRE software with CFR21 Part 11 functionality database, particle statistics and reporting function |
| Standards | Inbuilt reference standards and automated performance qualification |
| Qualification | Full IQ/OQ/PQ |