Analyse pharmaceuticals with Raman microscopy
From drug discovery to formulation, development, and trouble shooting, our systems give you answers.
APIs, excipients and more
Renishaw's Raman systems have the performance and flexibility you need to analyse all your pharmaceutical materials.
- Identify and differentiate API polymorphs and excipients
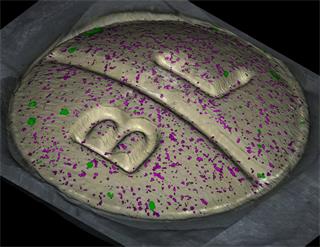
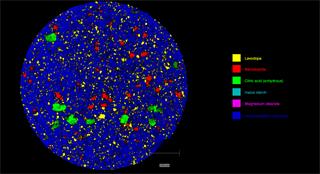
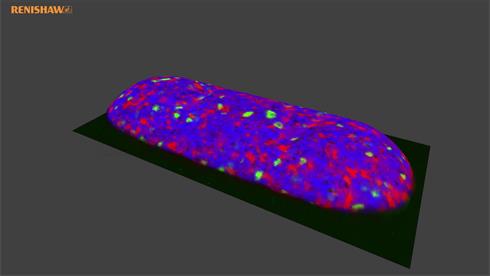
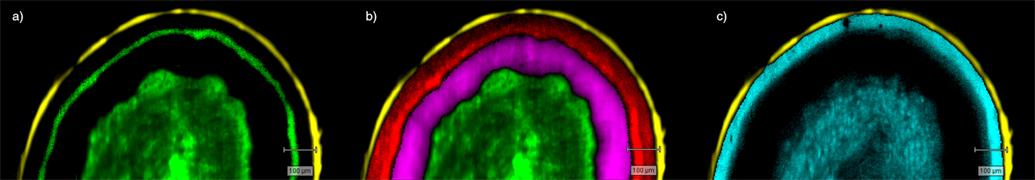
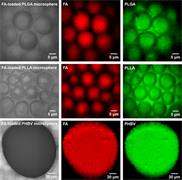
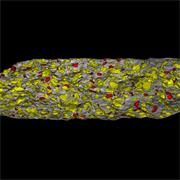
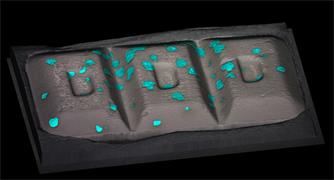
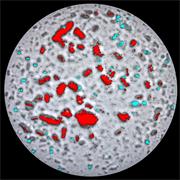
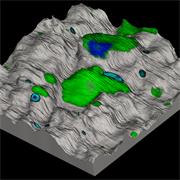
- Generate images of the formulations used in tablets, inhalers and nasal sprays at sub-micrometre spatial resolution
- Use our dedicated high-throughput polymorph screening capabilities
- Study the thermal behaviour of drugs using advanced temperature control
- Fast, quantitative analyses of entire tablets and powder blends
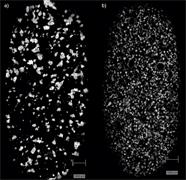
- Determine the uniformity of mixing
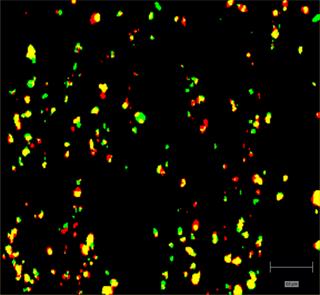
- Analyse API and metabolites in cells and tissue
- Identify contaminants
One spectrum reveals all
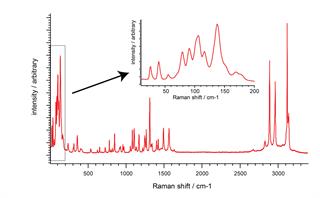
Save time. Use SynchroScan to reveal all the key Raman bands; at high spectral resolution, over the entire Raman range, in just one data collection. See lattice modes, OH bands, and everything in-between. Spot the most subtle sample differences wherever they occur in the spectrum.
Generate images rapidly
Rapidly generate images of your formulations with StreamLine™. This uses line focus illumination, allowing you to use higher laser powers without risk of sample damage, thereby reducing experiment times.
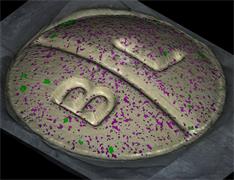
With the RA802 pharmaceutical analyser you can achieve imaging speeds of over 1500 spectra/s. It takes less than 2 minutes, from placing a standard tablet in the system, to generate a full, high resolution Raman image.
Generate images of formulations
Ensure your chemical images are representative; use Renishaw's StreamLine. You can change resolution to suit your domain size and, because Renishaw's WiRE software can cope with massive data files, you can analyse over the entire sample surface. Powerful Renishaw features, such as Slalom (to ensure the whole surface is sampled) and HD imaging (to get crisp clear images), provide all the options you need, whatever your formulation.
21 CFR 11
Renishaw's WiRE software supports 21 CFR part 11 compliance.
Product choices
RA802 Pharmaceutical Analyser
inVia™ confocal Raman microscope
Renishaw's RA802 system is designed exclusively for the pharmaceutical industry. The RA802 is a chemical imaging system optimised for routine analysis with the speed, automation and precision you need for reliable results. It is ideal for use in busy laboratories where the rapid analysis of multiple samples is required and space is at a premium.
Renishaw's inVia is a fully configurable research grade Raman microscope. Scientists worldwide trust inVia and its unparalleled flexibility. It can be upgraded, modified and customised, without compromising performance. Add accessories, lasers, fibre optic probes or combine with other analytical techniques. Whatever configuration you choose, you will have the most flexible Raman system on the market.
We're here when you need us
To find out more about this application area, or an application that isn't covered here, contact our applications team.
Contact our applications team