Denna sida finns för närvarande inte på ditt språk. Du kan
översätta
den automatiskt
med Google Translate. Vi ansvarar inte för att tillhandahålla denna tjänst och
vi har inte kontrollerat översättningsresultaten.
Kontakta oss om du behöver ytterligare hjälp.
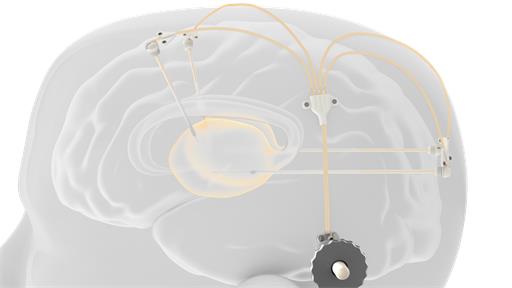
neuroinfuse™ drug delivery system
Novel chronic and acute catheter systems for direct delivery of therapeutics to the brain
Crossing the blood-brain barrier
Intraparenchymal drug delivery offers a practical method of bypassing the blood-brain barrier (BBB) and shows great promise in providing the next step change in the treatment of neurodegenerative, neuro oncology and other debilitating neurological conditions.
We are developing a patented range of engineering solutions to cover chronic (long term) implantable and acute (short term) implantable intraparenchymal drug delivery. Our low dead volume chronic device has a novel, MRI compatible, transcutaneous port that aims to provide a solution for simultaneous intermittent drug delivery to the central nervous system (CNS) through multiple catheters (up to four) at any time interval.
Features and benefits
Feature | Benefit |
| Implantation performed outside of MRI | Reduces the burden on costly MRI equipment and radiologists |
| MRI compatible | Enables real-time imaging of infusions |
| Acute infusions | Suitable for one-off infusions, catheter can be explanted after use |
| Chronic infusions | Enables repeated infusions and flexible administration regimes |
| Patient specific | Catheter length can be adjusted to encourage custom therapy distribution |
| 4-channel re-accessible catheter system with independent fluid paths and flow rates | Allows the clinician to control and customise the therapy regime |
| Repeat administrations do not require additional surgery | Reduces inherent risk of repeated surgery |
| Repeated delivery of fixed volumes | Aims to deliver consistent and repeatable treatment |
| Outpatient infusions | Reduces strain on in-patient facilities |
Watch an introductory video
A complete robotic platform
Working with clinical partners
Over several years Renishaw has been working with experienced clinical experts to produce a specification for an intraparenchymal drug delivery device and stereotactic delivery platform that facilitates convection enhanced delivery (CED) and other infusion applications.
The chronic product is currently undergoing clinical investigation as part of the Horizon 2020 funded TreatER project. TreatER is a first-in-human clinical study examining the intraparenchymal delivery of CDNF for the treatment of Parkinson's disease.
Drug development opportunities
At present, the Renishaw neuroinfuse chronic and acute drug delivery system can only be used in the setting of an approved clinical trial.
Renishaw is currently seeking academic, clinical and commercial partners across a wide range of indications, from oncology to neurodegenerative diseases.
The purpose of this website is only to obtain partners and not to make the device generally available.
Renishaw Neuro Solutions Limited – Legal and Privacy Notices. Click here