Denna sida finns för närvarande inte på ditt språk. Du kan
översätta
den automatiskt
med Google Translate. Vi ansvarar inte för att tillhandahålla denna tjänst och
vi har inte kontrollerat översättningsresultaten.
Kontakta oss om du behöver ytterligare hjälp.
Plant biology
Our understanding of plant biochemistry is fundamental to our ability to maintain ecosystems and provide sustainable nutrition.
Use Raman imaging to view the changes and distribution of metabolites, proteins, lipids, and pigments in studies of plant growth/development.
Determine plant health with Raman spectroscopy
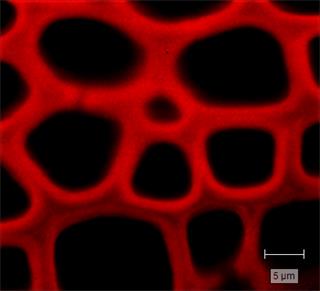
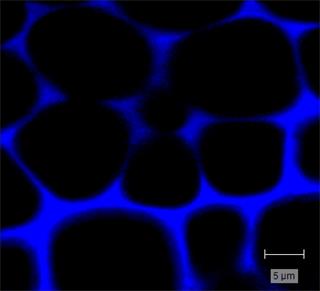
The thickness and distribution of the woody cell wall materials—cellulose, hemicellulose and lignin— are important factors for determining the strength of a plant. Raman imaging of cell walls is one of the only techniques that can be developed into an in-situ lignin and cellulose characterisation and quantification method. It does not require maceration of the materials or any wet chemistry and has the spatial resolution to resolve the thin lignin layer (sub-micrometre). An increase in lignin content is normally related to increased strength, with more guaiacyl lignin at the cell wall, and more syringyl lignin at the junction between the cell walls.
Pectin can also be Raman imaged. In terrestrial plants, it is found in the cell walls and is the major component in the middle lamella keeping cell walls together.
You can also study cellulose fibres, including their orientation (using polarised Raman measurements). The orientation is important because the angle of the microfibril affects the cell geometry and the plant's architecture.
Key benefits of Raman analysis
You can:
- image important biomolecules in situ without labelling
- quantify wood components in situ without the need of external labels
- resolve the structural and mechanical properties of wood and pulp, and the secondary order of lignin
- determine the orientation of wood fibres, which affects the cell geometry and architecture of plant
Sample preparation
Plant sections can be prepared and mounted on slides according to the protocol in Gierlinger and Schwanninger (Gierlinger and Schwanninger (2006). Plant Physiology 140: 1246-1254). They used PEG as an embedding material to allow sectioning.
Transparent or semi-transparent samples—such as sapling roots—may be analysed directly. You can use a cover slip, with water, to keep them hydrated.
Leaf/grass epidermis may be analysed by making an incision across the sample and peeling off the epidermal layer using forceps/tweezers. The same cover slip method will keep the epidermal layer hydrated.
We're here when you need us
To find out more about this application area, or an application that isn't covered here, contact our applications team.
Contact our applications team
Related stories
A healthy look at plants Raman imaging of plant chemistry
It is vital that we monitor plants and detect changes taking place, ideally before the visible signs of them occur. We therefore need suitable analytical techniques. Raman spectroscopy is ideal for this.
inVia aids discovery of rare mineral in alpine plants
The rare mineral, vaterite, has been discovered in plants for the first time by a team at Sainsbury Laboratory Cambridge University.